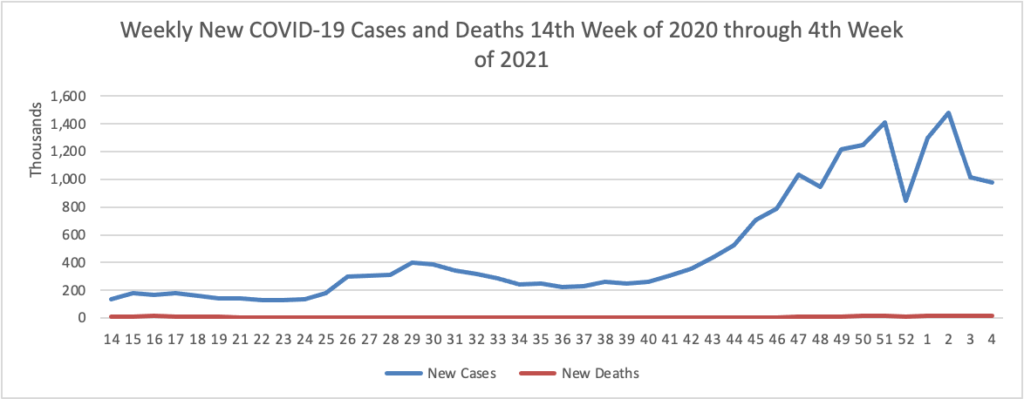
Based on the Centers for Disease Control’s COVID Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 4th weeks of this year (beginning April 2, 2020, and ending January 27,2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases greatly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2020 through January 27, 2021):
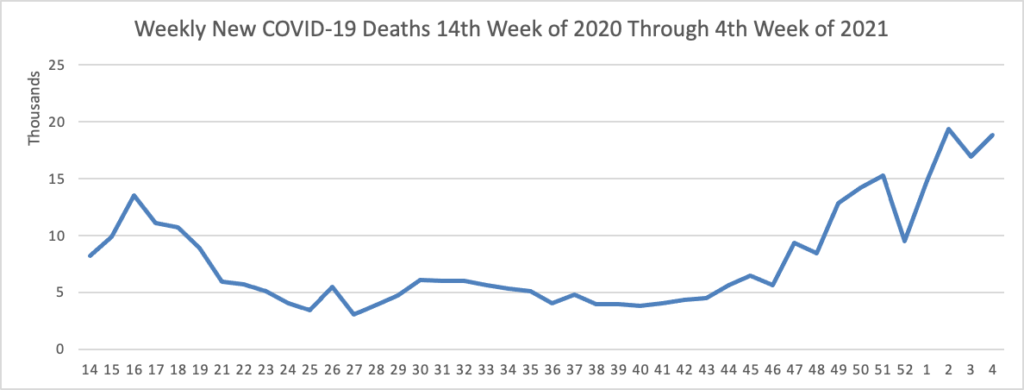
Finally here is a COVID-19 vaccinations chart for the past six weeks which also uses Thursday as the first day of the week:

Nearly 1.7 million doses of the COVID-19 vaccine were administered yesterday.
STAT News reports that ” The [Johnson & Johnson single dose COVID-19] vaccine reduced severe disease alone by 85%, and prevented Covid-related hospitalization or death, Johnson & Johnson said. “In a pandemic, if you can, with a single-dose vaccine, very quickly eliminate the severe consequences of death, hospitalization, and severe disease, that’s what’s important for society,” Paul Stoffels, the company’s chief scientific officer, told STAT. * * * Johnson & Johnson expects to file with the FDA for an emergency use authorization in early February, and, assuming the vaccine is authorized, will have some product ready to ship immediately after getting a go-ahead.” That’s great news.
The Paper Gown explains how to help people overcome vaccination fears.
The CDC’s FluView reports that “Seasonal influenza activity in the United States remains lower than usual for this time of year.“
The CDC today called attention to its Antibiotic Resistance Investment Map.
The Health Affairs Blog offers information on “suicide risk and protective factors, highlights current suicide prevention strategies, and notes policy opportunities for improving multisectoral prevention efforts. “
On the regulatory freeze front,
In accordance with the memorandum of January 20, 2021, from the Assistant to President Biden and Chief of Staff, entitled “Regulatory Freeze Pending Review,” and given the pendency of litigation, Pharmaceutical Care Management Association v. U.S. Department of Health and Human Services, et al., Civil Action No. 21-95 (JDB) (D.D.C.), challenging the final rule, HHS has released an action that temporarily delays for 60 days from the date of the memorandum the effective date of the final rule titled “Fraud and Abuse: Removal of Safe Harbor Protection for Rebates Involving Prescription Pharmaceuticals and Creation of New Safe Harbor Protection for Certain Point-of-Sale Reductions in Price on Prescription Pharmaceuticals and Certain Pharmacy Benefit Manager Service Fees,” published in the November 30, 2020, Federal Register. The effective date is delayed until March 22, 2021.
The anti-rebate rule would only affect federal government health plans, e.g., Medicare Part D, which are subject to the Federal Health Programs anti-kick back act. The FEHBlog is thankful that Congress exempted the FEHBP from this law when it was expanded beyond Medicare in 1996. The anti-rebate rule should be withdrawn.
