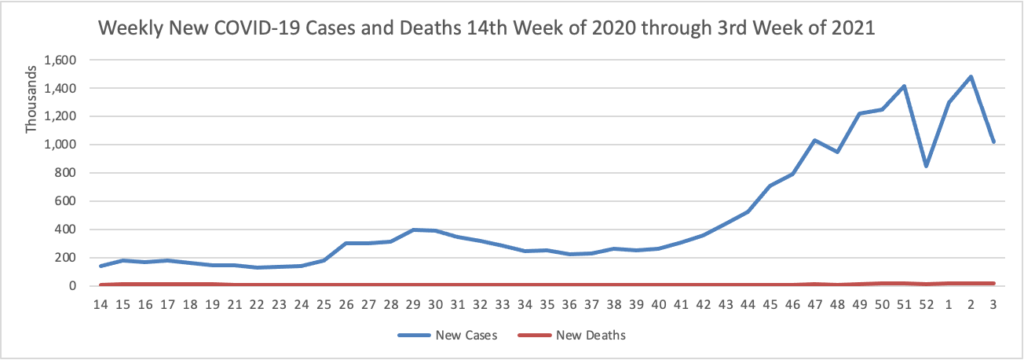
Based on the CDC’s Cases in the U.S. website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through third week of this year (beginning April 2, 2020, and ending January 20, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

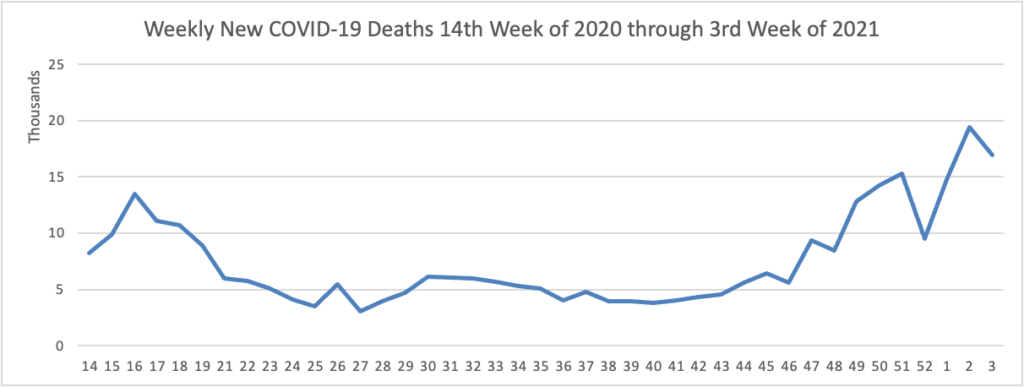
The FEHBlog has noted that the new cases and deaths chart shows a flat line for new weekly deaths because new cases greatly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2, 2020, through January 20, 2021):
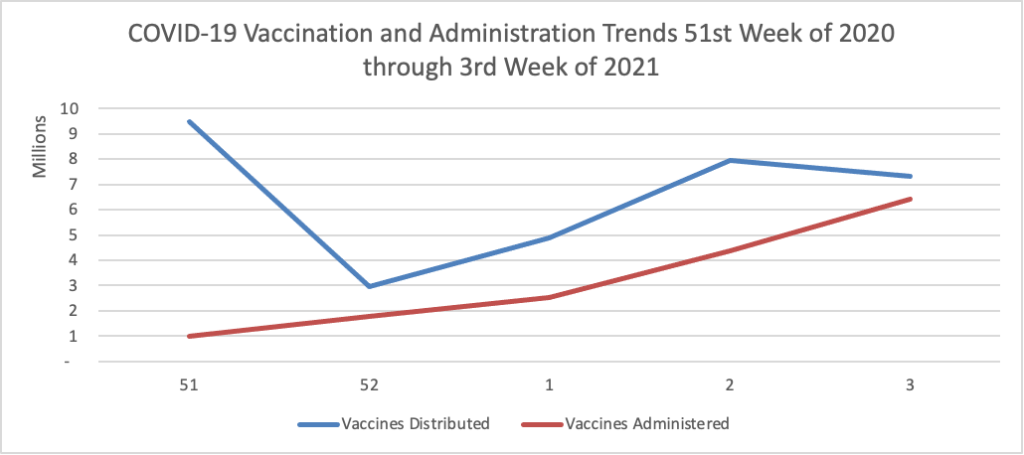
Finally here is a COVID-19 vaccinations chart for the past month which also uses Thursday as the first day of the week:
Here’s more from the COVID-19 vaccine front —
Bloomberg reports
As of Thursday, 940,000 shots a day were administered on average over a seven-day period, according to data from the Bloomberg Vaccine Tracker. The most recent two days topped a million doses.
Anthony Fauci, Biden’s chief medical adviser, said on Thursday that vaccinating 70% to 85% of the country by the end of the summer would enable a return to normalcy. To do so would mean administering 460 million to 560 million doses, since the current vaccines require a first shot followed by a booster. That’s more than double the rate of Biden’s 100-day goal.
The President’s goal cannot be considered a stretch goal and the FEHBlog is not suggesting that he had been doing so. Bloomberg adds
The U.S. spent more than any country in the world to help speed up the development and deployment of [COVID-19] vaccines. It secured more than 1 billion doses from six companies before any of the shots had been approved. For all the criticism that has been directed at the early fumbles of the vaccine rollout, the U.S. still leads the world in shots administered and is fifth in the world on per-capita basis.
The American Hospital Association reports in its daily email about “rare” levels of allergic reactions to the COVID-19 vaccines:
The Centers for Disease Control and Prevention today reported 10 cases of anaphylaxis, a life-threatening allergic reaction, among the more than 4 million people who received a first dose of the Moderna COVID-19 vaccine between Dec. 21, 2020, and Jan. 10, 2021. That’s an estimated 2.5 cases per 1 million doses. Nine cases occurred within 15 minutes of vaccination and one after 30 minutes. All of the individuals received emergency treatment with epinephrine; four were treated in an emergency department and six were hospitalized. All eight for whom follow-up information was available reportedly recovered. On Jan. 6, CDC reported 21 cases of anaphylaxis among the nearly 1.9 million people who received a first dose of the Pfizer COVID-19 vaccine between Dec. 14 and 23. That’s an estimated 11.1 cases per 1 million doses, but still a rare outcome, according to Nancy Messonnier, M.D., director of CDC’s National Center for Immunization and Respiratory Diseases. CDC recommends that locations administering COVID-19 vaccines adhere to its guidance, including screening recipients for contraindications and precautions, implementing recommended post-vaccination observation periods and immediately treating suspected anaphylaxis with intramuscular epinephrine injection.
Bloomberg reports on second dose timing:
People may receive their follow-up doses of the Covid-19 vaccines up to six weeks later if it’s not feasible to get them in the recommended interval, the Centers for Disease Control and Prevention said.
The guidance posted in a Jan. 21 update to the CDC website said a second dose should be administered as close to the recommended schedule as possible, either three weeks for the Pfizer Inc.–BioNTech SE vaccine or four weeks for the Moderna Inc. shot.
But if it’s impossible to get the follow-up shot on time, the CDC says people may schedule it as much as six weeks, or 42 days, after their initial dose. There is “limited data on efficacy” of the vaccines beyond that interval, according to the guidance, but if the second dose is administered later “there is no need to restart the series.”
Bloomberg adds
Vaccine supply and the pace of vaccinations could accelerate rapidly when new shots are cleared for use. A vaccine by Johnson & Johnson has enough data to begin analyzing now, and could have results in a week or two, Fauci said this week. The Food and Drug Administration has moved within days to authorize vaccine applications based on early results.
The U.S. has secured contracts with J&J for enough vaccine to inoculate 100 million people—the same as its agreements with Pfizer and Moderna. Unlike the two vaccines that are currently available in the U.S., J&J’s vaccine requires only one dose — which could accelerate the pace of vaccinations. It also can be stored in standard refrigerators, unlike Pfizer’s vaccine, which requires special freezers to keep temperatures below -94 degrees Fahrenheit.
Two other vaccines, from AstraZeneca Plc and Novavax Inc., have yet to be cleared for use in the U.S. but could add hundreds of millions of additional doses to the effort to stop the pandemic.
In other encouraging COVID-19 vaccine news, FiercePharma reports that “Americans are back on the COVID-19 vaccine bandwagon. Sixty-nine percent now plan to get a COVID-19 vaccine, close to the previous high of 73% in April, according to the latest data from The Harris Poll.”
