Based on the Centers for Disease Control’s Covid Data Tracker and using Thursday as the first day of the week, here is the FEHBlog’s weekly chart of new Covid cases from the 27th week of 2021 through the 24th week of 2022:
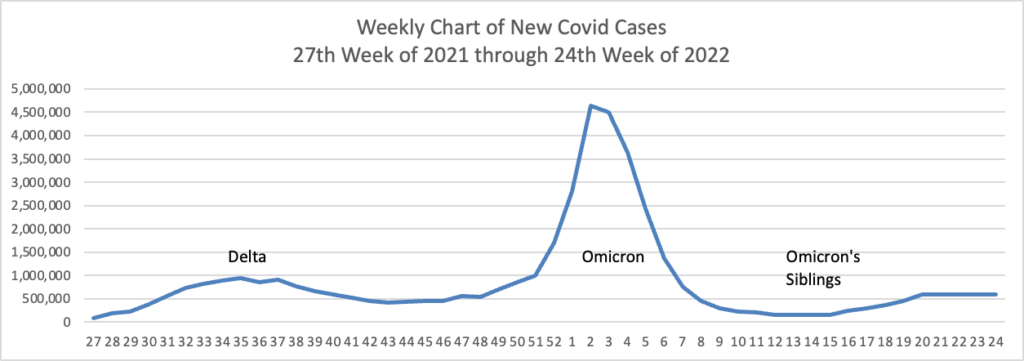
While the CDC did not publish its weekly Covid statistical review this week, the FEHBlog can tell you that the daily new Covid hospitalization average is 4,321 for the week ended June 15, 2022. That average is 4.7% higher than the previous week.
Here’s the FEHBlog’s weekly chart of new Covid deaths for the same period as new cases:
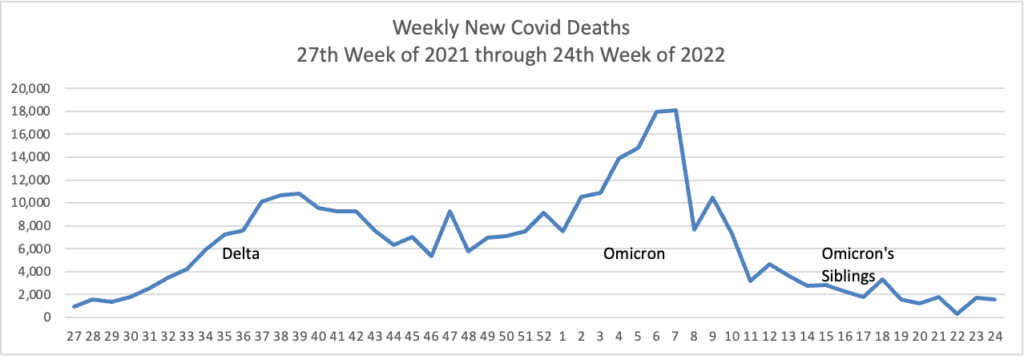
New cases and deaths are both stable. MedPage Today adds
About 4.5% of people who became infected with SARS-CoV-2 when Omicron was the dominant strain experienced long COVID symptoms, compared with 10.8% who became infected during the Delta period, reported Claire Steves, PhD, of King’s College London in England, and co-authors.
Overall odds of long COVID were about 20% to 50% less during the Omicron era — defined as December 2021 to February 2022 in this study — depending on age and time since vaccination, the researchers wrote in a letter to The Lancet.
For more information, Science discusses clues to long Covid.
Here’s the FEHBlog’s weekly chart of new Covid vaccinations distributed and administered from the 51st week of 2020, when the Covid vaccination era began and the current 24th week of 2022.
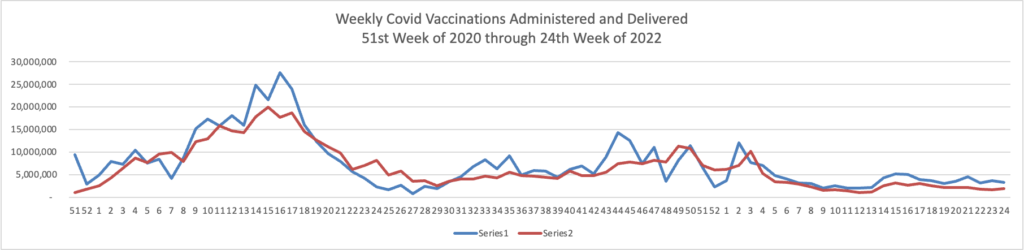
Over 100,000 people aged 12 and older have received at least one booster.
The Wall Street Journal adds.
Vaccine advisers to the Centers for Disease Control and Prevention discussed Friday the merits of Covid-19 shots now authorized for children as young as 6 months. * * *
The committee is scheduled to discuss further the case for recommending giving the Pfizer-BioNTech and Moderna shots to young children, and then vote on the matter Saturday.
The Biden administration has said the shots could be made available as soon as Monday, should regulators give their assent.
The federal government has begun shipping millions of doses to doctor’s offices, children’s hospitals and pharmacies, President Biden said after the FDA’s authorizations.
From the Rx coverage front, STAT News and Medical Economics update us on the federal government’s investigation of prescription benefit managers. Notably, STAT News reports
Amid intensifying scrutiny of pharmacy benefit managers, a group of House Republicans is urging the U.S. Government Accountability Office [via a June 17 letter] to investigate the role these controversial middlemen play in the opaque pharmaceutical pricing system.
From the Federal Trade Commission front, Healthcare Dive tells us that the FTC has obtained several victories over the past week in its legal challenges to hospital system mergers in New Jersey, Rhode Island, and Utah.
From the No Surprises Act front, reginfo.gov informs us that on June 15, the Office of Information and Regulatory Affairs received for review the NSA regulators’ final rule on the independent dispute resolution process. OIRA review is the last step before the rule is published in the Federal Register. The healthcare provider associations mounted a successful legal challenge to the interim final rule’s treatment of the qualifying payment amount as a rebuttable presumption at the arbitration stage.
From the HIPAA standard transactions front, a Health and Human Services workgroup has issued guidance on how payers can help providers associate electronic explanations of benefits with electronic payments.
From the U.S. healthcare front, Kaiser Health News and a WTW study delve into the widespread related problems of medical debt and deferred care. On the bright side, the National Institutes of Health reports that flexible work schedules and paid sick leave improves employee access to healthcare.
