From Capitol Hill, Govexec reports that “The Senate on Thursday cleared 65-27 a three-week stopgap bill to avoid a government shutdown, sending the measure to President Biden with just one day before the deadline.”
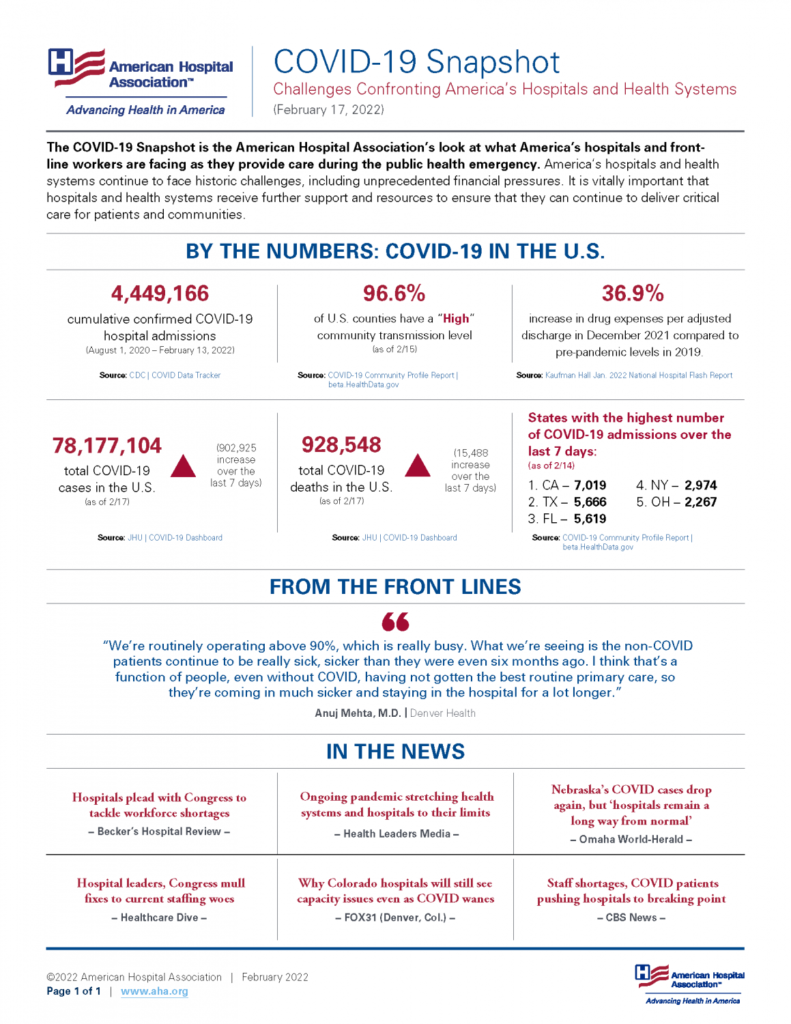
From the Omicron front, the American Hospital Association provides us with its most recent Covid snapshot
From the Covid treatment front, The American Medical Association reports
Efforts to boost production of [Covid] therapeutics are underway, a Food and Drug Administration (FDA) official said during an episode of the AMA-sponsored webinar series, “COVID-19: What Physicians Need to Know.”
Recommended usage in therapeutics is an important step in counseling patients and providing the most timely and relevant information about defeating this virus, said AMA President Gerald E. Harmon, MD, who moderated the webinar. Physicians facing supply issues of hard-to-get antivirals such as Paxlovid (PDF) also want to know about the timeline of any new antivirals or antibody treatments, he said.
For doctors and other health professionals, “it’s been a very difficult winter and it’s not over yet,” acknowledged John Farley, MD, MPH, director of FDA’s Office of Infectious Diseases in the Center for Drug Evaluation and Research’s Office of New Drugs. Monitoring the situation with these drugs is a complex process that involves FDA, the Centers for Disease Control and Prevention and several branches of government, he added.
Distribution of therapeutics should improve over time. However, at least for the combination of antiviral nirmatrelvir and ritonavir tablets marketed as Paxlovid, “we have a period of continued short supply ahead,” said Dr. Farley, who joined two other FDA experts to discuss the efficacy of the treatments.
In the FEHBlog’s view, Pfizer’s Paxlovid appears to offer the best opportunity for lowering Covid hospital admissions which have been a major cost for health plans over the past year which has seen three major Covid surges.
Speaking of innovations, Cleveland.com tells us
The next generation of mRNA vaccines, as well as treatments for type 2 diabetes and postpartum depression are among the innovations that earned spots on the Cleveland Clinic’s Top 10 Medical Innovations for 2022.
The list of breakthrough technologies, chosen by a committee of Clinic experts, was announced Wednesday.
These medical advancements have the potential to transform healthcare in the coming year, the Clinic said. The committee considered technologies developed by the Clinic as well as other research centers.
From the Federal Trade Commission front, Healthcare Dive reports
The Federal Trade Commission will not launch a study into pharmacy benefit managers’ pricing and contractual practices after a 2-2 vote at a Thursday meeting. The measure needed a simple majority to commence the investigation, which would have compelled large PBMs to turn over information and documents to the agency.
The two commissioners appointed by former President Donald Trump, Noah Phillips and Christine Wilson, voted against the study after raising concerns about its design and whether the current draft asks the proper questions for the answers the agency is seeking. Phillips also complained about receiving a “substantially revised” draft from staff “just hours” before the meeting.
FTC Chair Lina Khan said she was disappointed with Thursday’s vote, and said this is an area the agency has “a real moral imperative” to act upon.
From the Rx coverage front, Fierce Healthcare informs us
Optum has launched a new solution for specialty drugs that aims to lower costs and improve care management for people with complex conditions.
Specialty Fusion arms payers and providers with real-time insights into which specialty therapies are the most effective for the patient at the lowest cost. The platform leads to quicker treatment approvals for patients as well as a similar experience for providers at the point of care, Optum said in an announcement.
Internal analysis of the solution suggests it can drive cost savings of 17%.
From the healthcare business front, Healthcare Dive notes
Pharmacy giant Walgreens and value-based medical network VillageMD are on pace to open more than 200 co-branded primary care practices by the end of the year.
On Wednesday, the companies opened their first clinic in a new Florida market, Jacksonville, bringing their total markets in the state up to three, including Orlando and Tampa. Walgreens and VillageMD plan to open five new Village Medical at Walgreens primary care practices in total in the Jacksonville area through this summer.
With the Jacksonville openings, Walgreens and VillageMD have now opened more than 80 primary care practices across 12 markets in Arizona, Florida, Texas, Kentucky and Indiana.
Last but not least FedWeek explains how to guard against losing FEHB coverage in retirement.
