Based on data from the Centers for Disease Control’s COVID Data Tracker, here is the FEHBlog’s weekly chart of new COVID cases for 2021:
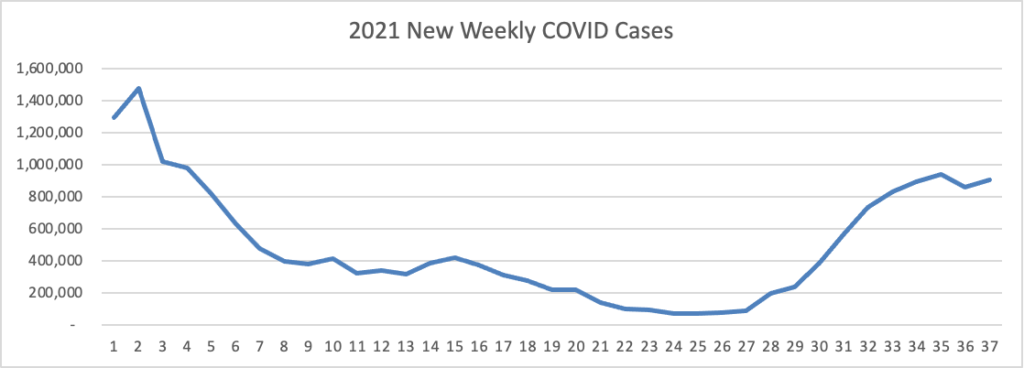
The CDC observes “After experiencing a brief decline in COVID-19 cases, the United States is once again seeing an increase in cases in most of the country.”
Here’s a link to the CDC’s weekly chart of COVID hospitalizations which shows a drop in the seven day moving average.
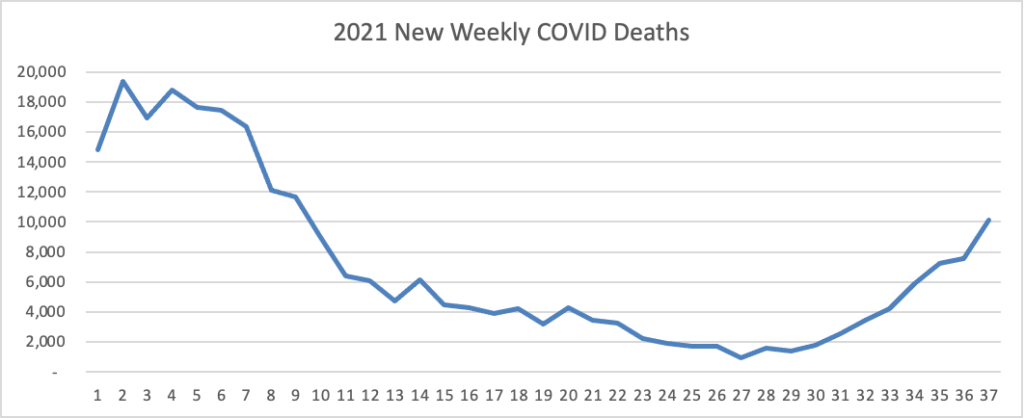
Here’s the FEHBlog’s weekly chart of new COVID deaths for 2021:
Finally here is the FEHBlog’s weekly chart of COVID vaccines distributed and administered from the 51st week of 2020 through the 37th week of 2021, ending last Wednesday September 15.
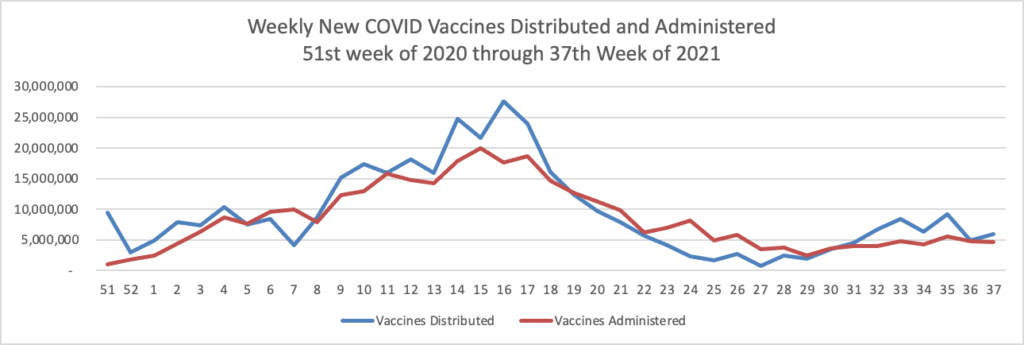
There are 180.6 million fully vaccinated Americans, or 63.6% of the vaccination eligible population (age 12 and older) and 31 million more who are waiting for their second dose.
The Wall Street Journal informs us that
The Covid-19 vaccine made by Moderna Inc. is more effective at keeping people out of the hospital than those from Pfizer Inc. and partner BioNTech SE or Johnson & Johnson, new research indicates.
In a study published by the Centers for Disease Control and Prevention on Friday, researchers studied more than 3,600 adults who were hospitalized in the U.S. between March and August of 2021. They looked at people who were admitted to 21 hospitals who had at least one Covid-19 symptom and a positive PCR or antigen test, as well as patients who were admitted to a hospital who tested negative for Covid-19. They then compared their vaccination status and which vaccine they received.
The researchers found that the Moderna vaccine’s effectiveness against hospitalization was 93%, compared with 88% for Pfizer-BioNTech’s and 71% for J&J’s. The effectiveness of the Moderna and Pfizer-BioNTech vaccines also seemed to stand up better over time than that of J&J’s vaccine. The Moderna vaccine’s effectiveness against hospitalization dropped to 92% after 120 days, while Pfizer-BioNTech’s dropped to 77%. After just 28 days, the J&J vaccine’s effectiveness fell to 68%.
And the administrative suspense is over, STAT News tells us
An advisory panel to the Food and Drug Administration on Friday recommended against a booster dose of a Covid-19 vaccine for most Americans at this time — a major rebuke to the Biden administration — but voted unanimously to recommend one to Americans who are 65 or older.
The FDA is not required to follow the recommendation of its advisory committees but generally does. If the recommendation is adopted by the FDA and Centers for Disease Control and Prevention, it would put the U.S. policy on a par with countries like the United Kingdom.
After seven hours of deliberation, members of the Vaccines and Related Biological Products Advisory Committee voted 16 to 2 against a proposal to administer a third dose of the vaccine developed by Pfizer and BioNTech to individuals 16 years and older. The vote to recommend a booster to people 65 years and older — as well as people who are at risk of severe Covid — was 18 to 0.
It was not immediately clear who would qualify as high risk; fleshing that out will likely fall to the CDC’s advisory committee, the Advisory Committee on Immunization Practices.
You will be able to knock the FEHBlog down with a feather if acting FDA Commissioner Janet Woodcock overrules the advisory committee given the Aduhelm debacle earlier this year. Also the outcome strikes the FEHBlog as a reasonable Goldilocks solution to the thorny problem.
Speaking of the expensive Alzheimer’s Disease drug Aduhelm, Fierce Pharma reports that
Data collected from a survey of 74 neurologists collected at the start of the month found that at least two thirds of respondents anticipate having at least some patients on Aduhelm by March 2022, according to Spherix Global Insights’ newly launched drug report released on Thursday. That could result in “an estimated brand share substantially higher than that projected back in August,” the report found.
he results indicate increased signs of optimism, according to Spherix, with small increases in the prescriber base and number of new patients compared to the stagnant trends found in the first few months following Aduhelm’s June FDA nod. In Spherix’s mid-July report, only about one fourth (27%) of responding physicians had planned to prescribe Aduhelm in the next few months.
Biogen’s prospects should improve in the coming weeks, Spherix estimates. According to the report, the prescriber base will likely grow by nearly 50% within that time, which could lead to twice the number of new patient initiations when compared with August.
Despite the maelstrom of negative press Biogen’s accelerated FDA nod has garnered, one in seven newly diagnosed patients with the memory-robbing disease are considered potential Aduhelm candidates, Spherix said.
Therefore, Aduhelm’s long-term success “does not appear to be markedly limited by physician willingness to prescribe” the treatment or “clinical determination of patient eligibility.”
In HHS News —
- Today, the Biden-Harris Administration announced a $2.1 billion investment to improve infection prevention and control activities across the U.S. public health and healthcare sectors. The Biden-Harris Administration, working through the Centers for Disease Control and Prevention (CDC), is investing American Rescue Plan funding to strengthen and equip state, local, and territorial public health departments and other partner organizations with the resources needed to better fight infections in U.S. healthcare facilities, including COVID-19 and other known and emerging infectious diseases.”
- Also today a record breaking third Affordable Care Act notice of benefit and rate parameters notice for 2022 was finalized. Fierce Healthcare tells us that “For 2022, insurers will face an increase in the federally run marketplace user fee rate to 2.75% of their premiums and the state-run exchanges to 2.25%. That is a change from the user fee finalized on Jan. 19 under the Trump administration, which called for a user fee of 2.25% for the federal marketplace and 1.75% for the state-run exchanges. The rule would also extend the open enrollment period by 30 days for ACA signups, with the new deadline on the federally run exchanges being Jan. 15. Open enrollment for the federal exchanges still starts on Nov. 1. * * * CMS is also creating a new special enrollment period that targets low-income individuals through HealthCare.gov. The goal is to target people who could be eligible for boosted subsidies that were included in the American Rescue Plan Act but expire after the 2022 coverage year. Congress is debating whether to extend those subsidies as part of a $3.5 trillion infrastructure package.”
